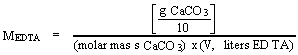Determination of Zn (II) by EDTA titration
Complexometric Titration of Zn(II) with EDTA
Complexometric Titration of Zn(II) with EDTA
Unknown Solution
Submit a clean 250-mL volumetric flask to the instructor so that an unknown zinc solution may be issued
Your name, section number, and your locker number should be written legibly on this flask
Note that the flask must be turned in at least 1 lab period before you plan to do the experiment, so that the Teaching Assistants will have time to prepare the unknown
Preparation of Solutions
USE ONLY DEIONIZED WATER (NOT DISTILLED WATER!) IN THIS EXPERIMENT
EDTA 0.01 M
Dissolve about 3.8 g of the dihydrate of the disodium salt (Na2H2Y•2H2O) and 0.1 g MgCl2 in 1 liter of water
Store in a plastic bottle. A small amount of sodium hydroxide may be added if there is any difficulty in dissolving the EDTA
Try not to exceed 3.8 g of Na2H2Y•2H2O because much more than this dissolves only with difficulty
The EDTA solution should be filtered using suction filtration
See TA for the apparatus.NOTE: Break the suction before you turn off the water flow on the vacuum aspirator
Buffer pH 10
Each titration will require the addition of pH 10 ammonia buffer
The stock buffer solution has been prepared for you, and the appropriate quantity is dispensed directly into your titration flask from the “Repipet” repetitive dispenser located in hood #7
The buffer should be added immediately before you titrate a sample
Calcium Standard Solution
A Ca2+ solution is prepared as the primary standard. Obtain from the TA approximately 0.7 g of predried analytical-reagent-grade CaCO3
Accurately weigh a 0.25-g sample by difference into a 100-mL beaker
Add about 25 mL water and then add dilute HCl dropwise until the sample dissolves, then add 2 drops more
Mild heating will speed dissolution if necessary
Transfer quantitatively to a 250 mL volumetric flask (rinse well with water) and carefully dilute to the mark. (eye dropper!) Mix thoroughly
Because this Ca2+ standard solution is used to standardize the EDTA titrant, it must be prepared very carefully so you know its exact molarity, and therefore the exact (to ± 0.1 mg) mass of CaCO3 weighed out
Standardization of EDTA Solution
Pipet 25-mL aliquots of the standard Ca2+ solution into three or four 250-mL Erlenmeyer flasks
Remember that each aliquot contains one-tenth of the CaCO3 weighed out to prepare the standard solution
Take each sample to completion before starting next sample
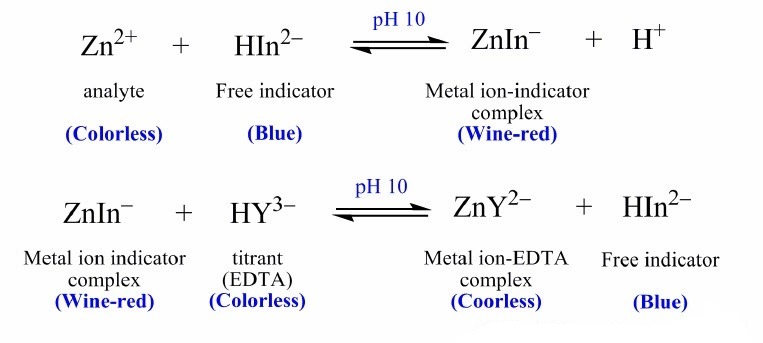
Add 7-8 mL of pH 10 buffer, 15 mL of water, and 3 drops of Eriochrome Black T and titrate immediately with EDTA until the light red solution turns a LIGHT SKY BLUE
Titrations must be performed swiftly (but carefully) because ammonia will evaporate and thus the pH of the solution will change
In general, the faster the titrations are performed the better the results will be (as long as the endpoint is not overshot due to the speed)
It is advantageous to perform a trial titration to locate the approximate endpoint and to observe the color change
In succeeding titrations, titrate very rapidly to within about 1 or 2 mL of the endpoint, then titrate very carefully (dropwise) to the endpoint. (See Note 1 at the end of the report.)
Calculate the molarity of the EDTA from the volume of EDTA used in the titration of each aliquot
The values (MEDTA and titration volumes) should all agree very closely. If not, titrate additional aliquots until agreement is reached, and any spurious values can be rejected with confidence
Determination of Zinc
Dilute your unknown sample in the 250-mL volumetric flask to the mark with water. Mix thoroughly
Pipet 25.00-mL aliquots into 250-mL erlenmeyer flasks
Add 15 mL of water, 9-10 mL of pH 10 buffer, and 2 drops of Eriochrome Black T immediately prior to titrating a sample
Titrate with standardized EDTA until the red solution turns blue. (See note at the end of the report.)
Calculate the number of milligrams of zinc in the total sample. Remember that each aliquot represents one-tenth of the total sample volume.
= MEDTA x (V, mL EDTA) x (molar mass Zn) x 10
Notes of Determination of Zn (II) by EDTA titration
Eriochrome Black T Indicator
The color change of Eriochrome black T at the endpoint is rather subtle
.It is not an abrupt chang from bright red to a dark blue; but rather it is from a light red (or pink) to a pale blue
.At least one trial titration is recommended. (You can always discard a “bad” value when you know there is a definite reason for its being bad
.If you have trouble distinguishing the endpoint, a “before” and an “after” flask are recommended
Prepare two 250-mL flasks exactly the same as for the samples, except use distilled water instead of a sample; add additional distilled water to approximately equal the volume of EDTA titrant that would be titrated into the flask for the sample
.Add the indicator. To one flask (the “after” the endpoint flask) add a small amount of EDTA to just past the color change at the endpoint
.Stopper the flasks and keep them nearby for comparison of the colors
.Titrate against a white background for better discrimination of colors (Extra flasks can be checked out from the stockroom)
.Sometimes the Eriochrome black T solution goes bad because of air oxidation
.If the endpoints seem very indistinct to you, try a fresh bottle of indicator
.Alternatively, try adding a small amount of solid Eriochrome black T mixture (1 g indicator ground with 100 g NaCl); a small amount on the end of a spatula is sufficient
pH 10 Ammonia Buffer
Dissolve 64.0 g of ammonium chloride in 600 mL of concentrated ammonia
Slowly and carefully add 400 mL deionized water
This should be sufficient for over 120 titrations
Reference: D. A. Skoog, D. M. West, F. J. Holler, and S. R. Crouch, Analytical Chemistry: An Introduction, 7th ed. Chapter 15, pp. 345-381